Human-Machine Interfaces(HMIs)-GOT GOT2000 Series

Security & Additional System Features
Support FDA 21 CFR Part 11
Regarding FDA 21 CFR Part 11 support

Having problems?
How can I support FDA 21 CFR Part 11 easily?
GOT will solve your problems!
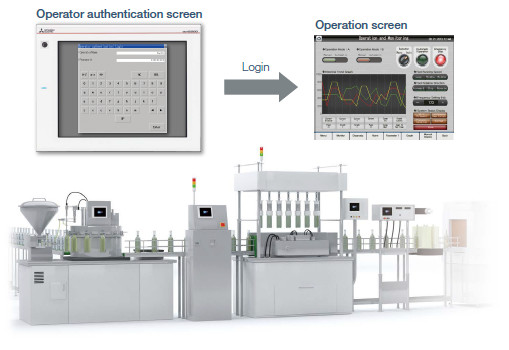
GOT can be used to make your system meet the requirements of FDA 21 CFR Part 11.
*The users must construct an appropriate system for the compliance with the FDA 21 CFR Part 11. For the details, please refer to the Technical Bulletin No.
Function features
GOT can be used to support FDA 21 CFR Part 11, the standards about electronic data recording of the traceability information, which is required in the food and pharmaceutical industries. Sample screens are available for helping you configure systems.
GOT functions related to FDA 21 CFR Part 11
- (1)Managing users who access the GOT
Operator authentication and security level setting - (2)Managing screen data
User management, access control - (3)Completeness of data
Network drive, FTP client, FTP server - (4)Security and viewing of data
Operation log, alarm, logging, recipe - (5)Audit trail
Operation log - (6)Validation of data and operations
Verification (GT Designer3 function) - (7)System development, operation, and management
Security level setting
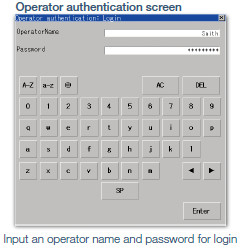
Access management per operator
The operator authentication function enables management of users who can login to GOT.
(See details of Operator authentication function)
*To prevent impersonations, user accounts should be managed thoroughly by the users.
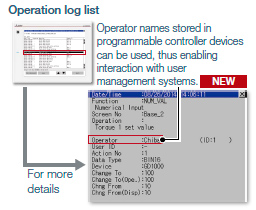
Recording audit trails (histories for the follow-up survey later)
Audit trails can be recorded and operated by setting the operation log appropriately.
(See details of Operation log function)
<Information required to be recorded>
- Time stamp
- User name of the logged-in operator
- Description and details of the operation performed by the operator
(logs before and after the data change)
Specification details and restrictions
●Range of supporting FDA 21 CFR Part 11
The range that GOT can support the regulation is limited. For the details, please refer to the Technical Bulletin No.
●How to obtain sample screens
Sample screens are included in GT Works3. For the details, please contact your local sales office.
The sample screens are supported by the following GT Works3 versions: Ver.1.152J or later.